Antifreeze at home
If you are the owner of a car, then you probably know that after the car has driven several tens of kilometers, it is necessary to fill the system with antifreeze fluid. You have to buy it all the time. But, there is a way to save money. And in our article we will tell you how to make antifreeze with your own hands.
What is this
Antifreeze or antifreeze is a non-freezing liquid that is poured into the expansion tank of a car. It is also used in heating systems. Translated in our language, antifreeze means “anti-freeze.” That is why it is also called anti-freeze. That is, this liquid does not freeze at subzero temperatures; it also cools the engine during operation and protects its elements from corrosion.
The production of antifreeze does not have a negative impact on nature. The composition of factory-made antifreeze often includes ethylene glycol, propylene glycol, glycerin mixtures, as well as monohydric alcohols and other water-based substances. Sometimes more flavorings are added. Note that it is quite easy to make antifreeze at home. How exactly? Read on!
We make antifreeze ourselves
Antifreeze at home is made from substances that are found in almost everyone. Therefore, you don’t have to buy expensive components.
What you will need
- isopropyl alcohol;
- washing powder;
- water (plain or distilled);
- ethylene glycol.
Process description
You might be surprised that homemade antifreeze contains alcohol. After all, it is clear that, for example, at a negative temperature on the windshield, the alcohol will evaporate, leaving only water, which itself will quickly freeze. But ethylene glycol saves everything here. It reduces the volatile property of the solution, so the alcohol does not have time to evaporate.
Often, a one to two ratio is used to prepare a solution, that is, one part alcohol and two water are taken.
This ratio can be changed if the temperature drops much below zero. At an air temperature of minus five degrees, a five-liter canister requires 0.6 liters of alcohol, at minus ten - one liter, at minus fifteen - 1.25 liters, at minus twenty - 1.65 liters, at minus twenty five - two liters, at minus thirty – 2.5.
Next, add ethylene glycol. You will have to calculate how much it is needed yourself. 15 cubic centimeters of ethylene glycol are required for each thermometer mark below zero.
Please note that ethylene glycol, like the antifreeze that contains it, is poisonous. If you are very concerned about your health, replace ethylene glycol with propylene glycol. It is much more expensive, but does not have toxic properties.
If desired, you can add natural flavoring to homemade antifreeze. It will remove the alcohol smell. You can use, for example, essential oil. By adding ten drops of it, you will rid the antifreeze canister of unwanted odor. We also note that you can enhance the cleaning properties of the liquid by simply adding a tablespoon of laundry detergent to it. If you adhere to all the above recommendations, then you will get antifreeze with your own hands no worse than the factory one.
Types of non-freezing liquid for heating
Factory antifreeze for heating
Having determined that non-freezing coolants for the heating system should only be of factory quality, you can begin to select a specific composition. It must be adapted to a specific heat supply scheme, and its performance indicators cannot worsen the parameters of the system.
Before pouring non-freezing liquid into the heating system, you need to find out whether it will negatively affect the heating components. To do this, you should read the instructions for use, which must be included. It is also important to pay attention to the main component of the antifreeze fluid for heating boilers. Not only the condition of the heat supply components, but also the operating conditions depend on this:
- Ethylene glycol . Characterized by high toxicity. Therefore, it can only be used in closed circuits. Difficulties may arise when pouring this type of freezing liquid into the heating system. In a vapor state, it is hazardous to human health;
- Propylene glycol . In fact, it is a food additive, so it can be used in both open and closed heating systems. In contrast to ethylene glycol, the crystallization temperature is +80°C, which makes it possible to use it to operate high-temperature solid fuel boilers. The only drawback is the high cost;
- Glycerin . The most popular type of non-freezing liquid for stove heating. Its performance qualities are slightly lower than those of propylene glycol. However, at the same time, the cost of glycerin antifreeze is an order of magnitude less. Disadvantages include high turnover. This may affect the tightness of the pipelines. The solution is to replace the rubber gaskets with paronite gaskets.
Currently, the use of non-freezing liquid for a home heating system based on glycerin is the best option.
| Name | Compound | Price, rub/l |
| Warm house -30°С | Propylene glycol | 65 |
| Dixis -65 | Glycerol | 75 |
| Coziness Technology -65 | Ethylene glycol | 120 |
Manufacturers offer 2 types of non-freezing coolants for heating systems - ready-to-use and concentrate. For large heat supply schemes, it is more profitable to purchase concentrate. However, this complicates the process of filling the system.
When purchasing ready-to-use liquid, you need to pay attention to the lower critical level of freezing temperature. It can be from -25°C to -65°C.
Antifreeze recipe: a secret behind seven locks?
Since ancient times, drivers have poured water into the radiator of their car. To prevent it from freezing, a special substance – ethylene glycol – was first added. The resulting mixture did not pose a danger to the cylinder block and radiator, since it turned into a viscous paste with small pieces of ice that were not prone to rupture of automotive equipment. For old cars that had cast iron engines and brass radiators, this substance seemed almost an ideal option, since it was also safe in terms of corrosion. This is how antifreeze appeared, which literally means “against frost.”
First problems
Along with the improvement of cars, the first troubles appeared. As it circulated through the new cooling systems, the heated antifreeze absorbed the metal. So the impeller and the walls of the cylinder head channels were left without whole pieces. Research institutes immediately began to solve this problem and proposed using additives in the form of inorganic salts to reduce corrosion activity. They contributed to the appearance on the walls of metal surfaces of a layer resistant to ethylene glycol.
At the same time, another name for antifreeze appeared - antifreeze. It appeared in a rather interesting way. The first three letters came from the department at the institute that dealt with the problems of improving antifreeze - “Organic Synthesis Technology”. The ending -ol is a tribute to chemical terminology. This is how the country first became acquainted with antifreeze!
So are there any differences?
We repeat - any coolant is antifreeze. Antifreeze is also antifreeze. Most sellers usually call antifreeze a liquid more intended for domestic cars. Although, even under the guise of “antifreeze” you can buy low-quality goods. To be confident in your choice, we recommend purchasing antifreeze only from well-known and trusted manufacturers. Preferably with reference to product endorsements by recognized automotive brands.
Detailed antifreeze recipe
Of course, not every driver can afford to buy expensive antifreeze from well-known manufacturers and suppliers. Most likely, this is why many car owners began to look for an antifreeze formulation in order to save financial resources. In reality, everything turned out to be not so simple. Large companies do not give away trade secrets, which, without a doubt, include product recipes. This is their profit - everything is logical and understandable here. I had to experiment for a long time.
But with the development of information technology, data has become more accessible. In particular, databases of patents from the times of the Soviet Union were posted on the Internet, where you can find the antifreeze recipe. Here we present just a few options. Anyone with access to the network can significantly expand this database
| Patent number | Component name | Content, % |
| Patent No. 2182585 | Ethylene glycol | 50-53 |
| Sodium benzoate | 4-6 | |
| Disodium phosphate | 1-1,18 | |
| Sodium nitrite | 0,1-0,13 | |
| Defoamer PMS-200A | 0,001-0,01 | |
| Fluorescein sodium salt | 0,005-0,01 | |
| Water | Rest | |
| Patent No. 1806162 | Distilled water | 33,41-41,92 |
| Ethylene glycol | 54,314-62,33 | |
| Sodium hydroxide | 0,791-0,912 | |
| Benzoic acid | 2,11-2,37 | |
| Borax | 0,622-0,713 | |
| Sodium nitrite | 0,121-0,142 | |
| Potassium nitrite | 0,03-0,034 | |
| Fluorescein sodium salt | 0,0007-0,0012 | |
| Defoamer PMS-200A | 0,0006-0,0013 | |
| Sodium metasilicate 9-water | 0,063-0,073 |
The data presented in the table should be considered rather as information for reference. Under no circumstances should you experiment with chemicals without having the necessary conditions for this.
If you have the starting conditions and know the antifreeze recipe, then you cannot do without special equipment designed for mixing antifreeze components.
First problems
Along with the improvement of cars, the first troubles appeared. As it circulated through the new cooling systems, the heated antifreeze absorbed the metal. So the impeller and the walls of the cylinder head channels were left without whole pieces. Research institutes immediately began to solve this problem and proposed using additives in the form of inorganic salts to reduce corrosion activity. They contributed to the appearance on the walls of metal surfaces of a layer resistant to ethylene glycol.
At the same time, another name for antifreeze appeared - antifreeze . It appeared in a rather interesting way. The first three letters came from the department at the institute that dealt with the problems of improving antifreeze - “Organic Synthesis Technology”. The ending -ol is a tribute to chemical terminology. This is how the country first became acquainted with antifreeze!
How to make antifreeze with your own hands
The “old guard” of motorists knows well what antifreeze is for and how it differs from imported antifreeze, but the younger generation will be curious to know about this coolant. In addition to these questions, we will also consider the rules for replacing it.
Domestic antifreeze - composition and labeling
This product is a liquid that circulates in the cooling system. It is also one of the varieties of antifreeze based on ethylene glycol, but only domestically produced. Its main tasks are to cool the engine in warm weather and prevent freezing of the working fluid in winter, as well as to protect the internal container from corrosion.
Depending on the labeling, the composition of the antifreeze, its density and, accordingly, its properties will vary. The following indices are used in the classification: A, M, K, 30, 40, 65. The letters indicate the type and are deciphered accordingly - automobile, modernized and concentrate. The numbers are the freezing temperature of this model of domestic antifreeze. The markings may also contain abbreviations for the names of manufacturers.
There are no coolants with a freezing point below 65 degrees Celsius, and it is the concentration of a substance such as ethylene glycol that provides different crystallization conditions. In addition to it, any, even domestic, antifreeze includes a set of additives, usually about 8–15, their purpose is to protect all pipes and components of the system from corrosion.
Antifreeze - technical specifications and purchase rules
Once at the counter in an auto store, most are perplexed as to which color of antifreeze is better, because they were not prepared for such variety. The answer is not that complicated! This parameter does not affect any quality characteristics; it is just a dye added by the manufacturer. Its main purpose is to differentiate brands and allow them to be mixed, for example, in model “40” the liquid is often blue, and “65” is red. It is also used for safety, since without dye, antifreeze has a transparent white color.
The boiling point of antifreeze usually lies in the range of 104–112 degrees Celsius; as for imported antifreeze manufacturers, it reaches 120–130. When purchasing, one of the ways to check quality is to measure the density; a good indicator is the limit from 1.060 to 1.090 g/cm 3 . Before going to the store, you need to determine what brand of coolant is in your car, and to do this, you should refer to the owner's manual. You can also find application rules there.
Leading manufacturers
Sometimes it is fundamentally important what kind of car you have, Toyota, Ford, Volvo, Renault or a car of another brand. Each manufacturer uses a cooling system with certain parameters and components adapted to work with a specific type of coolant.
Before purchasing any liquid, check the instruction manual. As for popular and well-known concentrates, they include the following mixtures:
It’s up to you to decide whether to use Castrol or Total. The main thing is that the composition is suitable for your engine. Be sure to take this into account.
The choice of fluid for the cooling system is made based on the operating conditions and recommendations of the manufacturer of the engine, and not the coolant.
Subscribe to our website, leave comments, ask relevant questions and tell your friends about our project!
( 2 ratings, average: 5.00 out of 5)
You can make antifreeze for heating yourself
- You can make your own radiator mixture. To do this you will need: distilled water and 40% ethyl alcohol.
To use antifreeze in a heating system, it is not always possible to fill in ready-made commercial mixtures. In this case, you can use a coolant that can be easily prepared with your own hands. you ask how is this possible? Everything is very simple, this coolant is a non-freezing mixture of distilled water and forty percent ethyl alcohol. Such antifreeze, prepared with your own hands for the heating system, has the following characteristics:
- the viscosity of the liquid is slightly higher than that of water, but much lower than that of commercial antifreeze;
- Alcohol-based mixtures have another advantage, making them an excellent choice for metal radiators. The fact is that alcohol prevents the development of corrosion, and this is important for systems whose destruction can lead to a variety of troubles;
- in this case, it is recommended to use hard water for the heating system, which, together with alcohol, prevents the formation of scale on the internal surfaces. The sediment is formed in solid form; during preventive flushing it is very easily removed from the system;
- when the alcohol content in the mixture is 30 percent or more, it does not evaporate separately;
- The boiling point of the alcohol coolant is approximately equal to the value for ordinary water. That is, when the temperature rises to 85 degrees Celsius, it does not boil with the formation of large masses of steam;
- alcohol in the coolant composition reduces thermal expansion, that is, when freezing, heating pipes and other elements are not damaged.
fluidity is less than antifreeze, which makes it possible to somewhat reduce the requirements for the tightness of circuit connections. Rubber seals are not damaged when using this particular liquid;
If you have to choose between water and an alcohol mixture, many experts recommend giving preference to the second option (if the boiler design allows this). The proportions of this composition are calculated based on what temperature values are planned:
If for some reason the boiler is turned off, be sure to drain the hot water from the radiator, otherwise the pipes may burst.
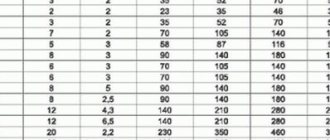
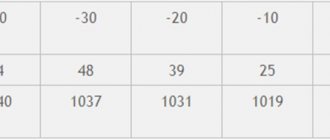
- when the temperature drops to minus 10.6 degrees, the alcohol content should be 20.3 percent;
- when it drops to minus 23.6 degrees, the alcohol content is 33.8 percent;
- when freezing to minus 28.7 degrees, the alcohol content should be 39 percent;
- when reduced to minus 33.9 percent, the alcohol content is 46.3 percent.
When preparing coolant for aluminum radiators, the volume must be calculated based on the fact that one liter of 96% ethyl alcohol contains 960 ml of anhydrous alcohol. To obtain a 33% alcohol solution, you need to divide 96 by 33, which will give a volume of 2.9 liters. When adding 2.9 liters of water to one liter of alcohol, we obtain a 33% alcohol solution, which is an excellent coolant, poured into an aluminum radiator for the heating system. The resulting solution will not freeze even at temperatures down to minus 22.5 degrees.
About antifreeze
The first coolant that consumers persistently want to pour into the heating system as an antifreeze agent is ethylene glycol, propylene glycol, or otherwise antifreeze or antifreeze. As the manufacturers assure, in the production of such coolant based on ethylene glycol, they use a special package of anti-corrosion, anti-foam, anti-scale and stabilizing additives (inhibitors). A mixture of water and ethylene glycol has the unique property of not freezing at low (down to -70 ° C) temperatures. What do you actually put into your heating system:
— The physical qualities of water-glycol solutions are lower than those of water: they are distinguished by high kinematic and dynamic viscosity and density. In order to prevent loss of pressure in the heating system, the user will have to either purchase a more powerful pump or increase the speed of the existing unit.
— Antifreezes have a greater coefficient of volumetric expansion than water. Therefore, when converting heating systems from water to a non-freezing coolant, they are equipped with expansion tanks of larger volume.
— The heat capacity of water-glycol solutions is lower than the heat capacity of water (usually by 15-20%), and they transfer heat from the boiler to the radiator worse. Therefore, in order for the efficiency of heat transfer by the system to remain the same, the user will have to either use more powerful radiators or speed up its circulation in the system (i.e. operate the pump more intensively).
One of the most important disadvantages of ethylene glycol is that it easily dissolves oil-based paint used to seal joints in heating systems. If there are minor cracks in the system, then ethylene glycol begins to leak through them, and also negatively affects the rubber gaskets in the system, through which it eventually begins to flow.
And finally, the most important thing is Ethylene glycol - Poison!
https://spirtprom.com.ua/
From our experience in the operation of heating systems using antifreeze
1. Antifreeze in the heating system must be changed at least every 5 years, otherwise they lose their properties and
many precipitate and clog the pipes; even high-pressure flushing of the pipes does not help; as a result, the pipes have to be replaced. There were such cases this year as well; pipes on two systems were partially replaced and were clogged with a clay-like mixture. 2. After about 5-6 years, all rubber gaskets in the heating system become unusable and leaks appear; the rubber melts under the influence of antifreeze and turns into dust. The next time you change antifreeze, you have to change all the rubber gaskets. Therefore, we recommend alcohol-based antifreeze to our customers and the price is cheaper. We have been using this antifreeze for 3 years now and there are no leaks from the systems.
Making your own antifreeze
Freezing of water is the main reason for using antifreeze.
It should be noted right away that ordinary water is the best type of coolant. It has sufficient heat capacity, has optimal density, and is affordable. Therefore, if the likelihood of exposure to negative temperatures affecting the heat supply is minimal, it is best to use distilled water.
But if this condition cannot be met, a special non-freezing liquid for heating boilers will be required. It is a solution in which water occupies up to 70% of the total volume. The rest is additives that reduce the crystallization threshold to -60°C. They include:
- The main component is ethylene glycol, propylene glycol or glycerin. This non-freezing liquid for the home heating system has a high viscosity coefficient, which leads to the desired effect;
- Additives . It is thanks to them that the non-freezing liquid for water heating does not foam and does not form a crystalline precipitate when the temperature rises.
The problem with making such a composition yourself is the correct selection of the last component. All manufacturers do not disclose the full list of components. But even knowing how to make the correct composition, it is impossible to do this at home - this will require special equipment and adherence to manufacturing technology.
How to make your own non-freezing liquid for heating, and what consequences can its use lead to?
- An increase in the level of foam during heating of the coolant will lead to the rapid formation of sediment on the walls of pipes and radiators;
- Reducing the heat transfer of homemade antifreeze liquid. This will cause a significant decrease in heating efficiency;
- Do-it-yourself non-freezing heating fluid can have a negative effect on the steel elements of the system due to the high oxygen content. Corrosion processes will accelerate.
Any non-freezing liquid for stove heating or solid fuel boiler should not cause these undesirable effects. Therefore, to maintain the safety of the system, it is recommended to use only high-quality antifreeze liquid for water heating from a reliable manufacturer.
Before using antifreeze, you should familiarize yourself not only with its composition and recommendations for use, but also carefully study the instructions for the heating boiler. It should indicate the types of coolant that can be filled.
Classic composition of antifreeze liquid
Using ordinary water to wash glass in frosty conditions is unsafe, so a special composition has been invented that remains in a liquid state at subzero temperatures.
Initially, alcoholic liquids were used for these purposes, and since perestroika, many variations of anti-freeze have been on the shelves of car dealerships.
Components contained in the composition:
- Water that performs the direct function of washing glass.
- Alcohol , usually isopropyl or ethyl. Previously, it was replaced by methanol derivatives, but now it has been proven to be unsafe for human health and the environment, so this component is officially banned. In reality, you can find counterfeits based on this ingredient, so untested products are unsafe.
- Surfactants or surfactants. Thanks to this component, you can quickly and without additional driver intervention remove greasy oil stains, dust and other contaminants from the surface of the windshield.
- Ethylene glycol keeps the solution liquid and prevents ice from forming.
- Denatured alcohols are used in ethyl alcohol-based liquids. This practice is adopted in order to avoid attempts to ingest liquid. This is a requirement of domestic regulations; there is no such thing in EU countries.
- Flavorings , the purpose of which is to wash away the possible residual smell of “chemicals” and present the product in an attractive way.
- Dyes provide visual appeal to the composition, as well as easy identification. Traditionally, washer fluid has a blue tint. Buying overly saturated compounds can lead to staining of the hood, so this factor should also be taken into account when purchasing.
Despite the large selection of purchased compounds, the question of how to make antifreeze with your own hands is as relevant as in times of total shortage.
The dubious primacy in falsifying automobile consumables belongs to glass fluid, so you can reduce the possible risk by preparing the composition yourself.
Such compositions are not perfect, but are available and tested over the years.
In what proportions should you mix water and alcohol for antifreeze?
The percentage of ethanol in the composition should be proportional to the air temperature outside the window.
Proportions of water and alcohol when making antifreeze
In addition, it is useful to remember that a liter of 96% ethyl alcohol, anhydrous alcohol, contains only 960 ml. Accordingly, to obtain a solution with 30% ethanol, you need 96/30 = 3.2, which means that for 1 liter of alcohol you need to take 3.2 liters of water. These proportions allow antifreeze not to freeze at temperatures down to -20o C.
Mixing
As a rule, concentrate manufacturers indicate on canisters how and in what proportions the composition needs to be diluted. Here everything directly depends on the temperature thresholds of the climate zone. For regions located in the middle zone, antifreeze with an indicator of -25 degrees is enough; for colder zones, at least -40 degrees will be required.
To prepare antifreeze with an indicator of -25°, concentrate and water must be mixed in a ratio of 2:3 (two liters of concentrated coolant and three liters of water). At the same time, the upper threshold drops from +196 degrees to approximately +130, +140, which is more than enough for many cars.
To prepare coolant with an indicator of -40°–45°, it is necessary to maintain a 1:1 ratio. In other words, the proportion of concentrate and water must be equal. The upper limit will be about 150-160°.
It should also be noted that there is no need to greatly dilute the concentrate with water, as this reduces the characteristics of not only freezing, but also overheating. For example, if you make a solution from one part of concentrate and four parts of water, you will get a lower limit of -15 degrees, and an upper limit of +100.
How to make antifreeze
You will need
- Antifreeze concentrate (OZh-K)
Instructions
To lower the freezing point of the coolant in your car's cooling system, add alcohol, for example, to the water. Or glycerin, which also helps lower the temperature at which crystallization of water molecules begins. But today such simple compositions of antifreeze coolant cannot solve all the problems that modern antifreezes face. Therefore, to make antifreeze, purchase antifreeze concentrate from the store and prepare it according to the attached instructions. Modern brands of antifreeze for cars with internal combustion engines are based on ethylene glycol. Ethylene glycol is a representative of polyhydric alcohols. When purified, it is a slightly oily, colorless, odorless, transparent liquid. It tastes sweet and is very toxic. Ingestion of ethylene glycol can be fatal.
In addition to ethylene glycol, additives are added to water to obtain high-quality coolant, which give antifreeze anti-corrosion properties. For this reason, leave antifreeze in the cooling system during the warm season. Moreover, ethylene glycol increases the boiling point of your coolant, and this is important in hot summer conditions.
Another important property that the coolant acquires thanks to additives is anti-cavitation. Cavitation is the formation and bursting of steam bubbles in a liquid when it boils. This process gradually destroys the metal, cutting microparticles from the surface and forming ulcers, which then corrode more easily. Ulcers grow into shells. Additives also give the liquid anti-foam properties.
note
The color of the coolant has nothing to do with the main characteristics of the antifreeze and does not in any way reflect its quality.
Is it possible to mix antifreezes and what does the color affect?
The question of antifreeze compatibility usually arises among car owners who have purchased a used car and are not able to determine the brand of liquid poured into the cooling system. Moreover, when solving this problem, car enthusiasts who do not understand the technical intricacies first of all take into account the color of the compound splashing in the expansion tank. And, indeed, manufacturers use dyes with a wide variety of shades to color coolants. The most popular colors: red, green, blue, yellow, purple, orange.
Some standards even regulate the use of certain shades. However, in fact, color is perhaps the last criterion that should be taken into account when mixing different brands of antifreeze. Dyes added to antifreeze are used only to make it clear that the liquid is technical, and, therefore, can threaten human health. In addition, thanks to the acquired tint, the visibility of antifreeze (initially colorless liquid) in the same reservoir of the cooling system improves. There is no direct connection between the color and properties of the coolant.
What considerations should be followed when mixing antifreeze? Here you can give at least a couple of tips:
- Without problems, you can combine antifreezes that have the same base and meet generally recognized quality standards. True, the composition of the liquid is often not published by the manufacturer, so all that remains is to follow the recommendations indicated on the label.
- Different types of antifreeze (with inorganic and organic additives) are allowed to be mixed only if the manufacturer clearly indicates this possibility.
The incompatibility of antifreezes lies in the likelihood of a reaction between the additives included in their composition. This may cause sediment to form or deteriorate performance, which may affect engine performance.
How to dilute antifreeze
You will need
Instructions
The basis for the production of antifreeze is ethylene glycol. An aqueous solution of the specified chemical substance with water in equal proportions is not inferior in frost resistance to undiluted liquid (within minus 40 degrees). As for anti-corrosion properties, diluted ethylene glycol, when heated, is up to two hundred times more aggressive towards metals than plain water. To minimize the activity of "Tosol", various additives (corrosion inhibitors) are added to it. It is precisely the difference between the chemical inhibitors added to Tosol and foreign-made antifreezes in the form of additives that distinguish domestic and imported antifreeze liquids, which are strictly prohibited from mixing. Otherwise, in one winter you can cause such damage to the car that it will require replacing the cooling and heater radiators, the water pump, and sometimes the engine cylinder head.
It is also necessary to emphasize the following: Russian “Tosol A40” is an aqueous solution of ethylene glycol in a ratio of 45:53:2. (45 – distillate, 53 – ethylene glycol, 2 – additives).
Thanks to additives (corrosion inhibitors), the water jacket in which “Tosol” circulates is covered with a protective film that preserves the surface from corrosion, and the addition of any amount of distillate to it is contraindicated, contrary to widespread belief about the possibility of such mixing. If you don't want to damage the engine cooling system, then never do anything like that.
Taking into account all of the above, only Antifreeze A40 or its analogues can be added to the cooling system of cars filled with domestically produced antifreeze. It is usually topped up to the top mark of the expansion tank.
Antifreeze - we destroy myths, clarify and find out everything about this composition + video
The “old guard” of motorists knows well what antifreeze is for and how it differs from imported antifreeze, but the younger generation will be curious to know about this coolant. In addition to these questions, we will also consider the rules for replacing it.
This product is a liquid that circulates in the cooling system. It is also one of the varieties of antifreeze based on ethylene glycol, but only domestically produced. Its main tasks are to cool the engine in warm weather and prevent freezing of the working fluid in winter, as well as to protect the internal container from corrosion.
Depending on the labeling, the composition of the antifreeze, its density and, accordingly, its properties will vary. The following indices are used in the classification: A, M, K, 30, 40, 65. The letters indicate the type and are deciphered accordingly - automobile, modernized and concentrate. The numbers are the freezing temperature of this model of domestic antifreeze. The markings may also contain abbreviations for the names of manufacturers.
There are no coolants with a freezing point below 65 degrees Celsius, and it is the concentration of a substance such as ethylene glycol that provides different crystallization conditions. In addition to it, any, even domestic, antifreeze includes a set of additives, usually about 8–15, their purpose is to protect all pipes and components of the system from corrosion.
Antifreeze - technical specifications and purchase rules
Once at the counter in an auto store, most are perplexed as to which color of antifreeze is better, because they were not prepared for such variety. The answer is not that complicated! This parameter does not affect any quality characteristics; it is just a dye added by the manufacturer. Its main purpose is to differentiate brands and allow them to be mixed, for example, in model “40” the liquid is often blue, and “65” is red. It is also used for safety, since without dye, antifreeze has a transparent white color.
The boiling point of antifreeze usually lies in the range of 104–112 degrees Celsius; as for imported antifreeze manufacturers, it reaches 120–130. When purchasing, one of the ways to check quality is to measure the density; a good indicator is the limit from 1.060 to 1.090 g/cm3. Before going to the store, you need to determine what brand of coolant is in your car, and to do this, you should refer to the owner's manual. You can also find application rules there.
Typically the composition is designed for 3 years of use or approximately 80 thousand kilometers. Also, do not waste money on cheap homemade liquid; choose familiar logos on the packaging.
We will replace the coolant yourself
Despite numerous warnings, antifreeze and antifreeze can be mixed, but some details need to be taken into account. It is best if the liquids are the same color and from the same manufacturer; you can also merge different colors, although the probability of success here is small, but still possible. So, you need to mix them together and wait for some time. If sediment appears in the new mixture, then you risk ruining the system, and if there is none, you can pour it all into the tank.
The appearance of sediment is a signal that the compounds are incompatible, and their mixture may have dangerous properties for your car. Now about the replacement process itself. Under no circumstances should you open the radiator cap when the engine is hot. Park your car in a place where there is no slope, this will ensure 100% drainage of the waste fluid. To remove the old compound, in addition to the top one, you also need to unscrew the bottom cap on the radiator. Do not forget to first place a sufficient container under it for waste.
After flushing the entire cooling system and tightly screwing the lower outlet plug, the long-awaited replacement occurs, where antifreeze is poured in in this case, it’s easy to guess - this is the upper hole. We fill the system with liquid as much as possible, and then seal it. To do this, we start the car and monitor the temperature sensor, it should show the norm, after that we top up the composition again to the top, and the process is completed. After all the operations performed, it is important to monitor the heating of the engine for some time; it should not exceed the permissible values.
Features of pouring antifreeze into the heating system
In order not to make an antifreeze heating fluid yourself and at the same time risk the performance of the entire system, you need to purchase a ready-made composition. However, in addition to this, you should familiarize yourself with the filling technology.
If there is old coolant in the system, it should be drained. It is recommended to check its condition. The degree of contamination will indicate the relevance of comprehensive cleaning. This is done before adding antifreeze to the heating system. The subsequent stages of work consist of completing the following points:
- If antifreeze was used before , the system must be completely flushed. Otherwise, mixing two different antifreeze fluids for furnace heating may lead to undesirable chemical reactions;
- Closed system . In it, the filling point should be lower than all other heating devices. Using pumping equipment, the heating system of a private home is filled with non-freezing liquid. It is important that the pressure in the pipes does not exceed 3 atm;
- Open system . For it, the use of antifreeze liquid for water heating is not recommended. Constant exposure to air can lead to a significant increase in foaming. Filling is done through the upper expansion tank;
- Heating testing . The temperature in the system increases gradually. At the same time, the tightness of all components is checked, as well as the absence of extraneous noise during coolant circulation.
During operation, you will need to add antifreeze heating fluid yourself. Therefore, it is recommended to purchase it with a reserve - 15-20% more than the calculated volume of the system.
You cannot make your own non-freezing liquid for heating. The use of automotive antifreeze is also not recommended, since in most cases they are based on unsafe propylene glycol.